Clinical Study
Clinical Relevance of PCR Versus Culture in Urinary Tract Infections Diagnosis: Quantification Cycle as a Predictor of Bacterial Load

Pallavi Upadhyay, Arjuna Vallabhaneni, Edward Ager, Barbara Alexander, Adriana Rosato and Vijay Singh • Aug. 1, 2025
Originally published in: MDPI Journal, Volume 15, Issue 15
1. Introduction
Urinary tract infections (UTIs) have been routinely diagnosed by standard urine culture (SUC), which comes with inherent limitations as a diagnostic test. While SUC is considered the “gold standard” for UTI testing, cultures take time for incubation, identification and susceptibility testing of organisms recovered. Furthermore, it is reported that accuracy of SUC in identifying UTIs in symptomatic patients is approximately 60% [1]. Recently, advanced clinical diagnostic technologies, including expanded quantitative urine cultures (EQUC), next generation sequencing (NGS) and nucleic acid amplification tests (NAAT) such as quantitative polymerase chain reaction (qPCR) and real time qPCR, have been developed to address the low sensitivity and prolonged turnaround time of results associated with SUCs [2].
While each of these advanced technologies help address specific challenges of SUC, each also has unique impediments to routine clinical use. For example, EQUC methods enable detection of a broader range of viable bacteria and fungi, but do not improve testing turnaround time [3,4,5]. NGS-based testing provides greater sensitivity and accuracy; however, it is not widely used in routine UTI diagnosis [5], as the requirement of complex data analysis and specialized expertise for testing renders NGS-based UTI testing both time and cost-intensive [6]. qPCR-based molecular testing can overcome several of the challenges that SUC presents, including the ability to detect anaerobic, intracellular, and non-bacterial infections, accurate identification of all causative organisms (even those in low concentrations) in a clinical specimen, and with significantly faster turn-around time (often same-day results). Furthermore, a recent meta-analysis reported that qPCR had higher specificity and sensitivity for UTI pathogen detection compared with NGS [7]. However, despite the potential advantages of qPCR, one of the biggest challenges facing adoption of qPCR testing for UTI diagnosis in the clinical setting is its enhanced sensitivity, particularly when applied to a clinical specimen for which contamination with potential uropathogens is not uncommon. In this setting, quantifying the amount of organism recovered in an SUC has historically helped guide interpretation and treatment decisions.
With SUC, a quantitative threshold of ≥105 CFU/mL of bacteria is regarded as clinically significant in freshly voided urine. Bacterial counts between 104 and 105 CFU/mL are generally interpreted according to clinical status. Bacterial counts < 104 CFU/mL are considered to have lower probability of UTI [8]. Recent studies indicate that bacterial counts as low as 102 CFU/mL can cause UTI in symptomatic patients [9,10,11]. Thus, understanding how qPCR results correlate with that of traditional quantitative SUC results would be beneficial in helping to interpret the qPCR result in the context of the patient’s clinical presentations and symptoms, and ultimately, in understanding which pathogens may or may not require treatment.
To address this challenge, we performed real-time qPCR testing on previously cultured urine samples and compared (colony forming unit per milliliters [CFU/mL] versus quantification cycle [Cq]) for each sample to aid in PCR result interpretation and to enhance clinical applicability.
2. Materials and Methods
2.1. Bacterial Strains
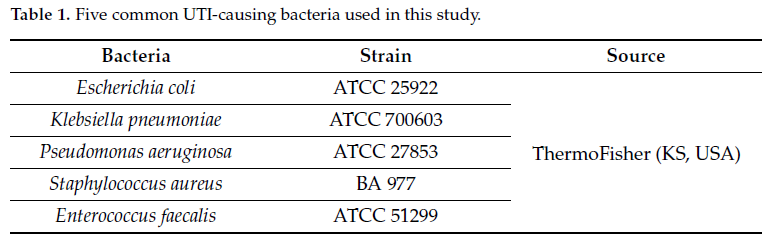
Bacterial strains (Table 1) were obtained from ThermoFisher (Lenexa, KS, USA) and were cultured on Tryptic Soy Agar (TSA) (Cat# R01624) or Mueller-Hinton Agar (MHA) (Cat# R111816) purchased from Remel (Lenexa, KS, USA).
2.2. Quantification of Bacterial Load in Urine Samples Using Culture-Based Methods
Contrived urine samples with various bacterial concentrations were prepared as follows. Using bacterial colonies grown overnight on TSA plates, an initial cell suspension equivalent to 0.5 McFarland (1.5 × 108 CFU/mL) was prepared in sterile urine by adjusting the cell density using a densitometer. A series of seven tenfold dilutions (1.5 × 107 to 1.5 × 100) were prepared in sterile urine. For each dilution, 100 µL was inoculated on either TSA or MHA in triplicate and incubated overnight at 37 °C. Colony counts were performed when discernible colonies were present; confluent growth was recorded as “too numerous to count” (TNTC). The experiment was repeated three times to ensure reproducibility.
2.3. qPCR to Determine the Cq Value for Known Bacterial Population Estimate
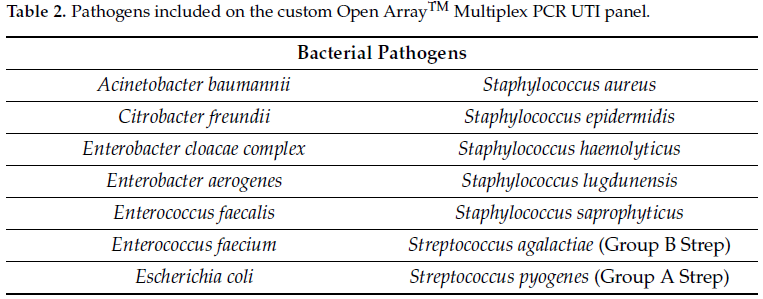
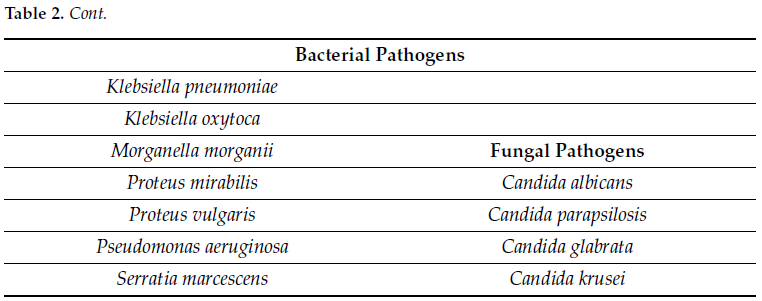
For each serial dilution, 1.0 mL was aliquoted in a 1.5 mL micro-centrifuge tube and centrifuged at 10,000× g for 10 min. The supernatant was decanted, and the cell pellet resuspended in 200 µL of PrimeStoreTM molecular transport medium (Longhorn Diagnostics, MD, USA). Total nucleic acid was extracted into 50 µL elution buffer using ThermoFisher KingFisher™ Flex (ThermoFisher Scientific, San Jose, CA, USA) system following the manufacturer’s protocol without any modification. Using 8 µL of extracted nucleic acid, qPCR was carried out using the HealthTrackRx OpenArray™ UTI syndromic panel (Denton, TX, USA) (Table 2) in triplicate for each dilution using the protocol described previously [12]. The OpenArray™ cards were run on ThermoFisher QuantStudio™ 12K Flex platform (ThermoFisher Scientific, CA, USA) with the following PCR cycling conditions: initial Enzyme activation at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 15 s followed by annealing/extension at 60 °C. The OpenArray™ technology is a high-throughput nanofluidic platform that uses custom arrays pre-plated with TaqManTM assays for the detection of target pathogens via PCR. The performance of PCR assays with respect to limit of detection, sensitivity and specificity were performed according to well-established Clinical and Laboratory Standards Institute (CLSI) guidelines for multiplex PCR assays.
2.4. Establishing the Cq to CFU/mL Correlation Algorithm
Using results from the contrived urine specimens, and considering culture quantification as the standard, an algorithm was developed to correlate CFU/mL with Cq values as follows: the serially diluted bacteria were plated in triplicate at each dilution. Bacterial colonies were counted on each culture plate and counts were recorded. The average across three plates for each dilution was recorded and multiplied by a factor of ten to give CFU/mL. qPCR was performed in triplicate for each dilution to generate Cq values. The average of the three Cq values for each dilution was calculated. Standard curves for each pathogen target were established by plotting the Cq values against the CFU/mL data for each dilution. A correlational algorithm was then established to define a clinically relevant threshold of 105 CFU/mL based on Cq values obtained through qPCR according to the linear equation obtained through the standard curves.
2.5. Correlation of Cq and CFU in Clinical Specimens
Residual and de-identified clinical urine samples were obtained from ARUP Labs, Utah, USA. Samples were collected over a period of two months in a blind fashion to avoid any bias. No patient information was collected and only the concordance between the microbial culture and PCR results was calculated based on the detection of the pathogens. A study comparing the qualitative results of urine culture and qPCR for the specimen cohort was previously published [13]. In the current study, we retrospectively analyzed the concordance between Cq and CFU/mL in the 168 specimens for which both traditional urine culture data and Cq values using UTI syndromic qPCR were available. Correlation was performed for all bacterial isolates based on their Gram staining properties using the developed algorithm.
2.6. Statistical Analysis
Categorical variables are presented as numerical values (n, %). Error bars wherever presented depict the standard deviation. Statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Analysis, Vienna, Austria).
3. Results
3.1. Estimation of Colony-Forming Units from Serially Diluted Bacterial Cell Suspensions
To quantify bacterial load across serial dilutions, CFU/mL values were log-transformed (Log10) and plotted against the expected cell density based on turbidity measurements (Figure 1a). The dilution series began with a 0.5 McFarland standard, corresponding to 1.5 × 108 CFU/mL.
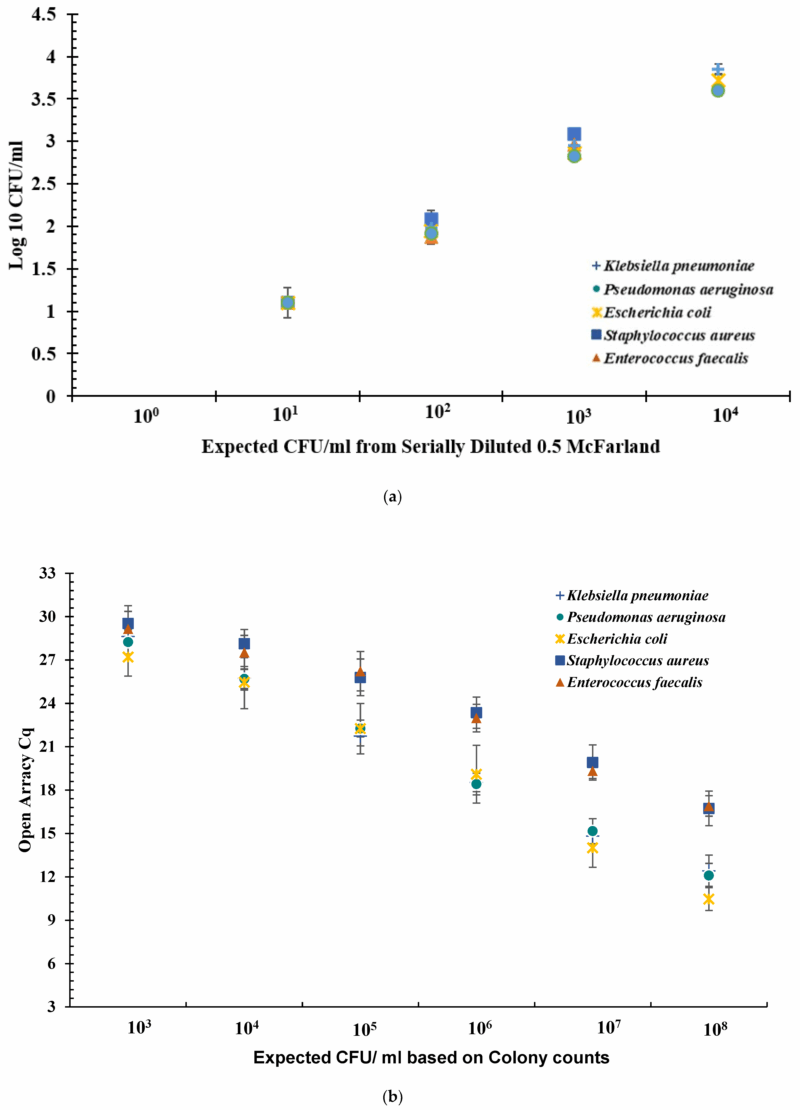
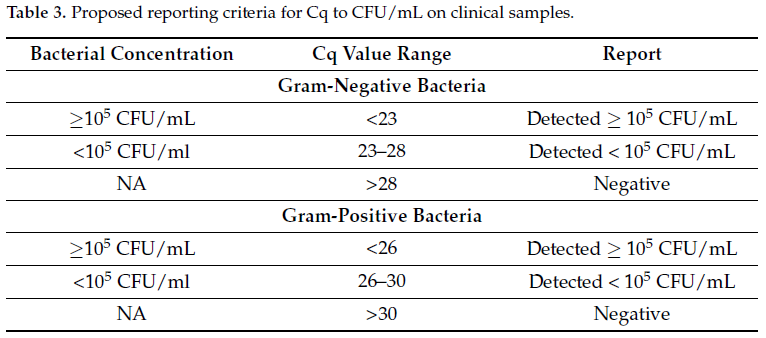
Figure 1. (a) Log10 CFU/mL for serially diluted bacterial cell suspension. The serially diluted bacteria were plated in triplicate at each dilution. All counts were recorded. The Average across three plates was recorded and multiplied by factor of ten to give CFU/mL. The value CFU/mL was transformed into Log10 value and plotted. The error bars indicate the standard deviation across three experiments of Log10 value of CFU/mL. Error bars represent standard deviation. (b) Open ArrayTM quantification cycle (Cq) values obtained at dilutions 103 to 108 CFU/mL for five bacteria spiked in urine. Sterile urine spiked with bacterial cells with concentrations ranging from 108 CFU/mL to 100 CFU/mL assayed for Cq values using qPCR. Each experiment was performed in triplicate. The data shown here is an average of three independent experiments. Error bars indicate standard deviation across three independent experiments. Error bars represent standard deviation.
Optical density measurements provided additional insight into bacterial concentration. When the expected Log10 (CFU/mL) value was 3.17, the average optical density for Gram-negative bacteria was 2.87 (±0.05), while for Gram-positive bacteria it was 3.02 (±0.03). At culture densities exceeding 105 CFU/mL, colony growth became too confluent for accurate enumeration, and these values were recorded as TNTC and excluded from further analysis.
3.2. Determination Cq Values by Open Array™ Multiplex qPCR
At bacterial cell densities of 104 and 105 CFU/mL, the following Cq values were observed: E. faecalis 27.5 (±1.9) and 26.2 (±2.4), S. aureus 28.1 (±1.3) and 25.7 (±2.4), E. coli 25.4 (±2.7) and 22.2 (±2.7), K. pneumoniae 25.7 (±1.4) and 21.7 (±1.4), P. aeruginosa 25.7 (±1.0) and 22.2 (±0.9), respectively. On average, Gram-positive bacteria showed distinctively different Cq values to that of the Gram-negative bacteria tested. In the case of Gram-negative bacteria at 105 CFU/mL, the average Cq value was 21.8 (±1.7), whereas for Gram-positive bacteria it was 26.5 (±2.4). Similarly, at 104 CFU/mL for Gram-negative bacteria, the average Cq was 25.6 (±1.6), whereas for Gram-positive bacteria it was 27.9 (±1.5) (Figure 1b).
3.3. Cq to CFU/mL Correlation Algorithm
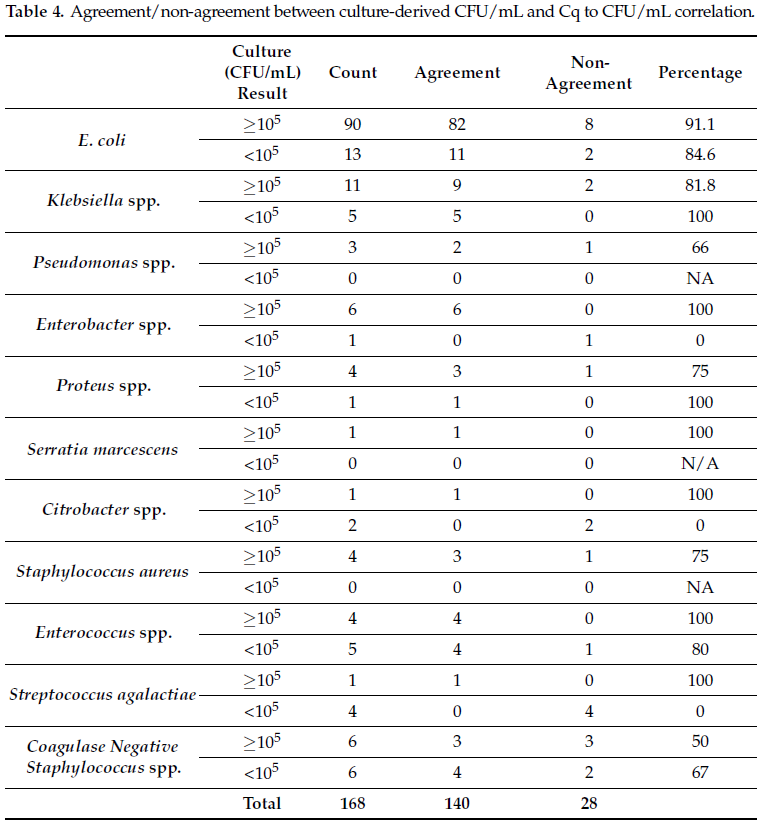
Based on the data from Cq to CFU/mL experiments (Figure 1a,b), a clinically relevant cutoff of 105 CFU/mL was determined on the basis of Cq values generated via qPCR. Per Table 3, for Gram-negative bacteria, Cq values of <23 corresponded with ≥105 CFU/mL and Cq values between 23 and 28 corresponded with <105 CFU/mL. For Gram-positive bacteria, Cq values of <26 corresponded to ≥105 CFU/mL and Cq values between 26 and 30, corresponded with <105 CFU/mL. With respect to the urine culture standards, Cq > 28 for Gram-negative bacteria and Cq > 30 for Gram-positive bacteria corresponded to negative.
We then retrospectively applied the Cq to CFU/mL criteria established using the three Gram-negative (E. coli, K. pneumoniae and P. aeruginosa) and two Gram-positive bacteria (S. aureus and E. faecalis) (Table 3) to the 168 residual clinical samples. Among 138 urine samples that grew Gram-negative pathogens, 116 resulted as ≥105 CFU/mL and among these, 89.6% (n = 104) displayed a Cq value of <23, which directly correlated to 105 CFU/mL range. In addition, of the remaining 12 Gram-negative bacteria that resulted as 105 CFU/mL, six had Cq values greater than 23 were all within the ±2 standard deviation (SD) observed in our study. Of 30 urine specimens that grew Gram-positive bacteria, 15 were quantified as greater than 105 CFU/mL and among those, 11 were below the PCR Cq value of 26. Overall, there was 83.3% agreement (n = 140/168) and 16.6% non-agreement (n = 28/168) between culture CFU/mL and Cq to CFU/mL. For Gram-negative isolates (n = 138), there was 87.6% (121/138) agreement and 12.3% (17/138) disagreement. Whereas, for Gram-positive bacteria (n = 30), the agreement was 63.3% (n = 19/30) and disagreement was 36.6% (n = 11/30) (Table 4).
The average Cq value detected for the five bacteria species used to develop the correlation algorithm all agreed with the proposed threshold values (Table 3). For E. coli the average Cq value detected in cultures reported as >105 CFU/mL was 17.99 (range 12.97 to 27.66). For Klebsiella the average value was 20.82 (range 17.11 to 23.89). The three Pseudomonas isolates gave an average Cq value of 22.61 (range 21.64 to 26.469). Two Enterococcus species detected had an average Cq value of 22.47 (19.94 and 25) and S. aureus Cq values averaged 21.26 (range 14.73 to 28.19). In cultures reported as <105 CFU/mL, thirteen E. coli were detected with an average Cq of 25.26 (range 18.69 to 28.46). The average for Klebsiella Cq values was 27.4 (range 23.51 to 19.25), and Enterococcus, 27 (26 to 29.7). The Cq-CFU/mL threshold algorithm developed as part of this study was also applied on Gram-negative and Gram-positive bacterial pathogens which were not part of the Cq-CFU/mL correlation, but that were detected by the multiplex PCR panel. A total of twenty-one isolates, sixteen Gram-negative and five Gram-positive bacteria were analyzed. Thirteen isolates showed positive agreement between reported CFU/mL values and CFU/mL values derived using the threshold values. All but one Proteus isolate (1/13) showed positive agreement for specimens reported as greater than 105 CFU/mL. Four S. agalactiae (GBS), one Enterobacter and two Citrobacter isolates were reported with CFU/mL values less than 105 CFU/mL which, following application of our proposed threshold values, the Cq values detected would have correlated to a CFU/mL value greater than 105 CFU/mL (Table 4, Figure 2).
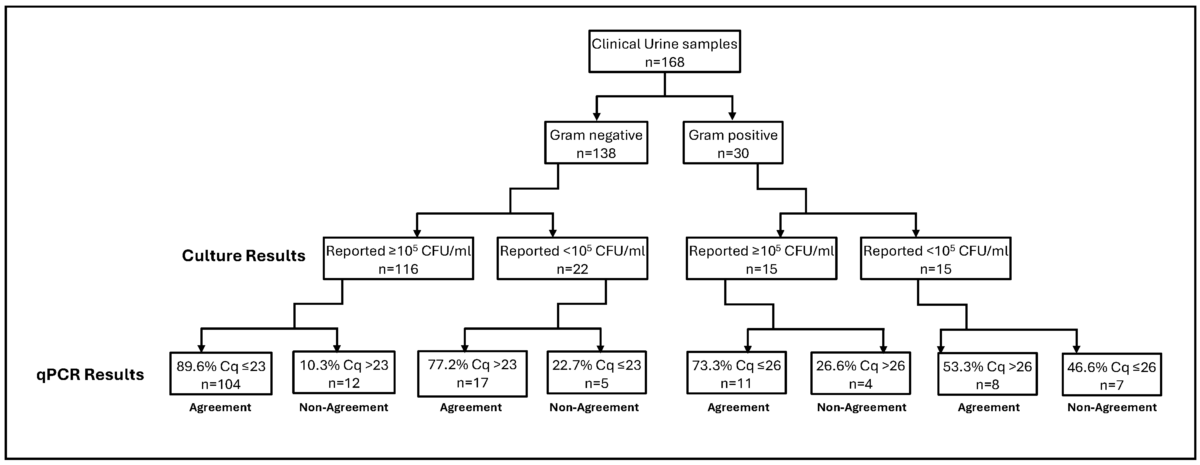
Figure 2. Cq-CFU/mL clinical validation. Cq values obtained from qPCR tests were compared to the previously tested urine samples via microbial culture. A Cq cutoff of ≤23 (for Gram-negative) and ≤26 (for Gram-positive) was used to establish correlation with culture results above or below 105 CFU/mL. The figure summarizes agreement/non-agreement between qPCR and microbial culture techniques reported in Table 4.
4. Discussion
To improve the clinical efficacy of multiplex qPCR-based UTI detection and to provide a tool for clinicians to reliably interpret UTI PCR results in relation to traditional culture results, this study aimed to correlate the multiplex UTI PCR values (Cq) with colony counts (CFU/mL) of urine cultures. Unlike molecular panels for other infectious syndromes, fewer studies have compared multiplex PCR to traditional urine culture for diagnosing UTIs [14,15,16]. For comparison, 100,000 CFU/mL (105) was set as the target comparator as this value is widely considered a clinical diagnostic cutoff for diagnosing UTIs in symptomatic patients [5].
The results from our study showed a CFU/mL value of ≥105 correlated with average Cq values below 22.5 for all Gram-negative bacteria assessed, whereas for Gram-positive bacteria the Cq value that best correlated to ≥105 CFU/mL was 26.5 (Table 3). Our results are in agreement with previously published studies wherein multiplex PCR-based detection of UTI-causing pathogens was directly compared to those detected via culture in a clinical setting [17]. In a similar comparative analysis of Cq and CFU/mL values for bacterial pathogens in bronchial samples, Burillo and co-workers reported 24.7 as the Cq value that best corresponded to 105 CFU/mL. The authors also described 104 CFU/mL as corresponding to an average Cq value of 26.9 for Gram-negative bacteria [18].
The derived semi-quantitative thresholds correlating Cq values to urine culture CFU/mL values were validated using a collection of clinical specimens previously submitted for urine culture and reported [13]. Based on the results of our Cq-CFU/mL study (Table 3), we used a Cq value of 23 for Gram-negative bacteria and Cq of 26 for Gram-positive bacteria as the semi-quantitative criteria to approximate a CFU/mL of ≥105. After converting Cq values to CFU/mL for previously tested urine isolates, we looked at the agreement between the two sets of CFU/mL values. The percent agreement between the results obtained after applying the threshold values was 83.3% (140/168) for the five bacterial strains used in this study (Table 4). Despite the overall high agreement, we observed 36.6% disagreement for Gram-positive bacteria. This can be attributed to differences in overall PCR efficiency, assay design and the small number of Gram-positive strains utilized to derive the Cq value scale which underscores the need for further studies with additional bacterial strains and qPCR assays to establish a more robust interpretation scale.
A major concern with PCR-based testing is the detection of pathogens in samples that are deemed culture negative [14,15,16], suggesting that PCR may be too sensitive a technique for reliably reporting clinically relevant levels of uropathogens. In general, the decision-making process of appropriate diagnostic tests for UTIs (based on clinical symptoms) as well as result interpretation for timely treatment remains a challenge [19]. Furthermore, the high sensitivity of molecular tests in comparison to traditional culture techniques complicates clinical interpretation of the results and remains a significant barrier in the widespread adoption of PCR for the detection of UTIs. Since PCR can detect nucleic acid material from non-viable and degraded pathogens, a positive PCR result may not reflect a true UTI even in symptomatic patients [20]. Similarly, in cases of a culture-negative but PCR-positive diagnosis, a clear distinction between infection and colonization cannot be made without further research into the clinical validity of the quantitative PCR test [17]. A study comprising 220 women with UTI symptoms demonstrated that 95.9% samples were positive for E. coli via PCR versus 80.9% cultures positive for any UTI-causing pathogen [17]. In our study, among the previously tested urine culture specimens, those reported as negative by culture were also analyzed. The multiplex UTI PCR panel included pathogens not readily detected in traditional culture, and several were observed in the study. Notably, there were 59 culture-negative specimens where E. coli was detected via PCR [13]. Upon applying our proposed threshold values for Cq-CFU/mL, 93.2% (n = 55/59) of these PCR-positive E. coli samples were interpreted to be less than 105 CFU/mL and only four had Cq values that would have been reported (≥105 CFU/mL). These findings suggest that our proposed semi-quantitative criteria, when applied to Cq values from patient specimens, reliably correlates to CFU/mL cutoff values used in the clinical diagnosis of UTIs and may help delineate pathogens from non-pathogenic and/or non-viable colonizers.
Previous studies have argued that PCR is a reliable and beneficial replacement for traditional urine culture [17,18,21]. Our study shows that a robust correlation between Cq and CFU/mL results exists, and the correlation can be effectively incorporated into result reports to assist in patient diagnosis and to enable more targeted approaches to antimicrobial therapy.
The implementation of syndromic PCR panels has had a significant impact on rapid disease diagnosis [22]. In support of NAATs, it has been opined that large multiplex PCR panels should be the first-line tests for detection of respiratory and intestinal pathogens [23]. A single-center, 150-patient study showed the standard urine culture (at 105 CFU/mL) displayed poor sensitivity and missed 67% of all detected UTI-causing pathogens, indicating a need for more sensitive methods for pathogen detection in urine specimens [3]. The increasing demand for high-throughput tests that can detect a range of diverse pathogens has further placed multiplex testing at the forefront of options sought by the modern clinical laboratory [2,24]. This testifies to the growing importance of multiplex PCR tests as an effective tool for uropathogen detection in clinical samples. Despite the increased sensitivity and specificity, a major disadvantage of PCR is its limitation of detecting only known resistance genes and the technology in its current format cannot be used for assessing true antibiotic sensitivity of a detected pathogen as opposed to established phenotypic susceptibility testing methods. However, when interpreted in conjunction with the clinical presentation and examination, qPCR tests may streamline testing, optimize antibiotic use and potentially improve patient outcomes [25,26].
We recognize that the current study has limitations. Firstly, the number of pathogens used to validate our Cq-CFU/mL threshold algorithm is limited. Further studies using more bacterial strains, particularly Gram-positive species, are currently being performed to support the observations made in this study. Secondly, the clinical validation on clinical samples was performed on residual samples. Another limitation of our study is the non-standardized nature of the PCR assay used, and the results cannot be universally utilized without incorporating multi-site testing. To further demonstrate the true clinical effectiveness of our correlation algorithm, prospective studies with defined patient populations are needed wherein urine culture is compared to the Open ArrayTM multiplex PCR panel simultaneously which would enhance the observations made in this study.
5. Conclusions
Molecular testing for UTIs, when compared to traditional culture techniques, presents advantages with respect to increased sensitivity and rapid turnaround time for results. However, widespread acceptance of this methodology is hampered by limitations of PCR-based results for establishing clinically relevant cutoffs of the detected uropathogens. Our results establish a clear agreement between the PCR-generated Cq value and the traditional CFU/mL value generated via microbial culture. Validation of our semi-quantitative interpretive scale using clinical samples demonstrates that PCR Cq values correspond with established standard of care quantitative culture thresholds, providing physicians with valuable additional information to aid in interpreting qPCR results for the diagnosis of UTIs.
References
- Yoo, J.-J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; Kim, W.B.; et al. Urinary Microbiome Characteristics in Female Patients with Acute Uncomplicated Cystitis and Recurrent Cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef]
- Szlachta-McGinn, A.; Douglass, K.M.; Chung, U.Y.R.; Jackson, N.J.; Nickel, J.C.; Ackerman, A.L. Molecular Diagnostic Methods Versus Conventional Urine Culture for Diagnosis and Treatment of Urinary Tract Infection: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2022, 44, 113–124. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Wolfe, A.J.; Brubaker, L. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef]
- Deen, N.S.; Ahmed, A.; Tasnim, N.T.; Khan, N. Clinical Relevance of Expanded Quantitative Urine Culture in Health and Disease. Front. Cell. Infect. Microbiol. 2023, 13, 1210161. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Deebel, N.; Casals, R.; Dutta, R.; Mirzazadeh, M. A New Gold Rush: A Review of Current and Developing Diagnostic Tools for Urinary Tract Infections. Diagnostics 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, I.; Aschbacher, R.; Pagani, E. Current Uses and Future Perspectives of Genomic Technologies in Clinical Microbiology. Antibiotics 2023, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qi, S.; Sun, Y.; Zheng, X. Comparison of Polymerase Chain Reaction and Next-Generation Sequencing with Conventional Urine Culture for the Diagnosis of Urinary Tract Infections: A Meta-analysis. Open Med. 2024, 19, 20240921. [Google Scholar] [CrossRef]
- Karah, N.; Rafei, R.; Elamin, W.; Ghazy, A.; Abbara, A.; Hamze, M.; Uhlin, B.E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings. Diagnostics 2020, 10, 832. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norby, S.R. Urinary Tract Infections: Disease Panorama and Challenges. J. Infect. Dis. 2001, 183, S1–S4. [Google Scholar] [CrossRef]
- Hooton, T.M.; Roberts, P.L.; Cox, M.E.; Stapleton, A.E. Voided Midstream Urine Culture and Acute Cystitis in Premenopausal Women. N. Engl. J. Med. 2013, 369, 1883–1891. [Google Scholar] [CrossRef]
- Kunin, C.M.; White, L.V.; Hua, T.H. A Reassessment of the Importance of “Low-Count” Bacteriuria in Young Women with Acute Urinary Symptoms. Ann. Intern. Med. 1993, 119, 454–460. [Google Scholar] [CrossRef]
- Singh, V.; Reddy, J.; Granger, J. A Survey of Viral-Bacterial Co-infection in Respiratory Samples Using Multiplex Real-Time PCR. J. Infect. Dis. Ther. 2019, 7, 400. [Google Scholar]
- Upadhyay, P.; Surar, F.; Kim, G.; Reddy, J.; Shakir, S.; Alexander, B.D.; Hanson, K.; Singh, V. Comparative Analysis of the Detection of UTI Pathogens via Culture Method and the Open Array-Nanofluidic Real Time PCR Method. Open Forum Infect. Dis. 2022, 9, ofac492. [Google Scholar] [CrossRef]
- Cybulski, Z.; Schmidt, K.; Grabiec, A.; Talaga, Z.; Bociąg, P.; Wojciechowicz, J.; Roszak, A.; Kycler, W. Usability Application of Multiplex Polymerase Chain Reaction in the Diagnosis of Microorganisms Isolated from Urine of Patients Treated in Cancer Hospital. Radiol. Oncol. 2013, 47, 296–303. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, A.; Roorda, L.; Bosman, G.; Ossewaarde, J.M. Molecular Diagnosis of Urinary Tract Infections by Semi-Quantitative Detection of Uropathogens in a Routine Clinical Hospital Setting. PLoS ONE 2016, 11, e0150755. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2020, 136, 119–126. [Google Scholar] [CrossRef]
- Heytens, S.; De Sutter, A.; Coorevits, L.; Cools, P.; Boelens, J.; Van Simaey, L.; Christiaens, T.; Vaneechoutte, M.; Claeys, G. Women with Symptoms of a Urinary Tract Infection but a Negative Urine Culture: PCR-Based Quantification of Escherichia coli Suggests Infection in Most Cases. Clin. Microbiol. Infect. 2017, 23, 647–652. [Google Scholar] [CrossRef]
- Burillo, A.; Marín, M.; Cercenado, E.; Ruiz-Carrascoso, G.; Pérez-Granda, M.J.; Oteo, J.; Bouza, E.; Floto, A.R. Evaluation of the Xpert Carba-R (Cepheid) Assay Using Contrived Bronchial Specimens from Patients with Suspicion of Ventilator-Associated Pneumonia for the Detection of Prevalent Carbapenemases. PLoS ONE 2016, 11, e016847. [Google Scholar] [CrossRef]
- Medina-Bombardó, D.; Jover-Palmer, A. Does Clinical Examination Aid in the Diagnosis of Urinary Tract Infections in Women? A Systematic Review and Meta-Analysis. BMC Fam. Pract. 2011, 12, 111. [Google Scholar] [CrossRef]
- Lehmann, L.E.; Hauser, S.; Malinka, T.; Klaschik, S.; Weber, S.U.; Schewe, J.C.; Stüber, F.; Book, M. Rapid Qualitative Urinary Tract Infection Pathogen Identification by SeptiFast Real-Time PCR. PLoS ONE 2011, 6, e17146. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Dumkow, L.E.; Worden, L.J.; Rao, S.N. Syndromic Diagnostic Testing: A New Way to Approach Patient Care in the Treatment of Infectious Diseases. J. Antimicrob. Chemother. 2021, 76, iii4–iii11. [Google Scholar] [CrossRef]
- Schreckenberger, P.C.; McAdam, A.J. Point-Counterpoint: Large Multiplex PCR Panels Should Be First-Line Tests for Detection of Respiratory and Intestinal Pathogens. J. Clin. Microbiol. 2015, 53, 3110–3115. [Google Scholar] [CrossRef]
- Huang, H.S.; Tsai, C.L.; Chang, J.; Hsu, T.C.; Lin, S.; Lee, C.C. Multiplex PCR System for the Rapid Diagnosis of Respiratory Virus Infection: Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2018, 24, 1055–1063. [Google Scholar] [CrossRef]
- Kelly, B.N. UTI Detection by PCR: Improving Patient Outcomes. J. Mass Spectrom. Adv. Clin. Lab. 2023, 28, 60–62. [Google Scholar] [CrossRef]
- Elia, J.; Hafron, J.; Holton, M.; Ervin, C.; Hollander, M.B.; Kapoor, D.A. The Impact of Polymerase Chain Reaction Urine Testing on Clinical Decision-Making in the Management of Complex Urinary Tract Infections. Int. J. Mol. Sci. 2024, 25, 6616. [Google Scholar] [CrossRef]
References
- Yoo, J.-J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; Kim, W.B.; et al. Urinary Microbiome Characteristics in Female Patients with Acute Uncomplicated Cystitis and Recurrent Cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef]
- Szlachta-McGinn, A.; Douglass, K.M.; Chung, U.Y.R.; Jackson, N.J.; Nickel, J.C.; Ackerman, A.L. Molecular Diagnostic Methods Versus Conventional Urine Culture for Diagnosis and Treatment of Urinary Tract Infection: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2022, 44, 113–124. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Wolfe, A.J.; Brubaker, L. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef]
- Deen, N.S.; Ahmed, A.; Tasnim, N.T.; Khan, N. Clinical Relevance of Expanded Quantitative Urine Culture in Health and Disease. Front. Cell. Infect. Microbiol. 2023, 13, 1210161. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Deebel, N.; Casals, R.; Dutta, R.; Mirzazadeh, M. A New Gold Rush: A Review of Current and Developing Diagnostic Tools for Urinary Tract Infections. Diagnostics 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, I.; Aschbacher, R.; Pagani, E. Current Uses and Future Perspectives of Genomic Technologies in Clinical Microbiology. Antibiotics 2023, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qi, S.; Sun, Y.; Zheng, X. Comparison of Polymerase Chain Reaction and Next-Generation Sequencing with Conventional Urine Culture for the Diagnosis of Urinary Tract Infections: A Meta-analysis. Open Med. 2024, 19, 20240921. [Google Scholar] [CrossRef]
- Karah, N.; Rafei, R.; Elamin, W.; Ghazy, A.; Abbara, A.; Hamze, M.; Uhlin, B.E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings. Diagnostics 2020, 10, 832. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norby, S.R. Urinary Tract Infections: Disease Panorama and Challenges. J. Infect. Dis. 2001, 183, S1–S4. [Google Scholar] [CrossRef]
- Hooton, T.M.; Roberts, P.L.; Cox, M.E.; Stapleton, A.E. Voided Midstream Urine Culture and Acute Cystitis in Premenopausal Women. N. Engl. J. Med. 2013, 369, 1883–1891. [Google Scholar] [CrossRef]
- Kunin, C.M.; White, L.V.; Hua, T.H. A Reassessment of the Importance of “Low-Count” Bacteriuria in Young Women with Acute Urinary Symptoms. Ann. Intern. Med. 1993, 119, 454–460. [Google Scholar] [CrossRef]
- Singh, V.; Reddy, J.; Granger, J. A Survey of Viral-Bacterial Co-infection in Respiratory Samples Using Multiplex Real-Time PCR. J. Infect. Dis. Ther. 2019, 7, 400. [Google Scholar]
- Upadhyay, P.; Surar, F.; Kim, G.; Reddy, J.; Shakir, S.; Alexander, B.D.; Hanson, K.; Singh, V. Comparative Analysis of the Detection of UTI Pathogens via Culture Method and the Open Array-Nanofluidic Real Time PCR Method. Open Forum Infect. Dis. 2022, 9, ofac492. [Google Scholar] [CrossRef]
- Cybulski, Z.; Schmidt, K.; Grabiec, A.; Talaga, Z.; Bociąg, P.; Wojciechowicz, J.; Roszak, A.; Kycler, W. Usability Application of Multiplex Polymerase Chain Reaction in the Diagnosis of Microorganisms Isolated from Urine of Patients Treated in Cancer Hospital. Radiol. Oncol. 2013, 47, 296–303. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, A.; Roorda, L.; Bosman, G.; Ossewaarde, J.M. Molecular Diagnosis of Urinary Tract Infections by Semi-Quantitative Detection of Uropathogens in a Routine Clinical Hospital Setting. PLoS ONE 2016, 11, e0150755. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2020, 136, 119–126. [Google Scholar] [CrossRef]
- Heytens, S.; De Sutter, A.; Coorevits, L.; Cools, P.; Boelens, J.; Van Simaey, L.; Christiaens, T.; Vaneechoutte, M.; Claeys, G. Women with Symptoms of a Urinary Tract Infection but a Negative Urine Culture: PCR-Based Quantification of Escherichia coli Suggests Infection in Most Cases. Clin. Microbiol. Infect. 2017, 23, 647–652. [Google Scholar] [CrossRef]
- Burillo, A.; Marín, M.; Cercenado, E.; Ruiz-Carrascoso, G.; Pérez-Granda, M.J.; Oteo, J.; Bouza, E.; Floto, A.R. Evaluation of the Xpert Carba-R (Cepheid) Assay Using Contrived Bronchial Specimens from Patients with Suspicion of Ventilator-Associated Pneumonia for the Detection of Prevalent Carbapenemases. PLoS ONE 2016, 11, e016847. [Google Scholar] [CrossRef]
- Medina-Bombardó, D.; Jover-Palmer, A. Does Clinical Examination Aid in the Diagnosis of Urinary Tract Infections in Women? A Systematic Review and Meta-Analysis. BMC Fam. Pract. 2011, 12, 111. [Google Scholar] [CrossRef]
- Lehmann, L.E.; Hauser, S.; Malinka, T.; Klaschik, S.; Weber, S.U.; Schewe, J.C.; Stüber, F.; Book, M. Rapid Qualitative Urinary Tract Infection Pathogen Identification by SeptiFast Real-Time PCR. PLoS ONE 2011, 6, e17146. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Dumkow, L.E.; Worden, L.J.; Rao, S.N. Syndromic Diagnostic Testing: A New Way to Approach Patient Care in the Treatment of Infectious Diseases. J. Antimicrob. Chemother. 2021, 76, iii4–iii11. [Google Scholar] [CrossRef]
- Schreckenberger, P.C.; McAdam, A.J. Point-Counterpoint: Large Multiplex PCR Panels Should Be First-Line Tests for Detection of Respiratory and Intestinal Pathogens. J. Clin. Microbiol. 2015, 53, 3110–3115. [Google Scholar] [CrossRef]
- Huang, H.S.; Tsai, C.L.; Chang, J.; Hsu, T.C.; Lin, S.; Lee, C.C. Multiplex PCR System for the Rapid Diagnosis of Respiratory Virus Infection: Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2018, 24, 1055–1063. [Google Scholar] [CrossRef]
- Kelly, B.N. UTI Detection by PCR: Improving Patient Outcomes. J. Mass Spectrom. Adv. Clin. Lab. 2023, 28, 60–62. [Google Scholar] [CrossRef]
- Elia, J.; Hafron, J.; Holton, M.; Ervin, C.; Hollander, M.B.; Kapoor, D.A. The Impact of Polymerase Chain Reaction Urine Testing on Clinical Decision-Making in the Management of Complex Urinary Tract Infections. Int. J. Mol. Sci. 2024, 25, 6616. [Google Scholar] [CrossRef]
Related Articles and White papers

Pallavi Upadhyay, Arjuna Vallabhaneni, Edward Ager, Barbara Alexander, Adriana Rosato and Vijay Singh • Aug. 1, 2025
Originally published in: MDPI Journal, Volume 15, Issue 15
1. Introduction
Urinary tract infections (UTIs) have been routinely diagnosed by standard urine culture (SUC), which comes with inherent limitations as a diagnostic test. While SUC is considered the “gold standard” for UTI testing, cultures take time for incubation, identification and susceptibility testing of organisms recovered. Furthermore, it is reported that accuracy of SUC in identifying UTIs in symptomatic patients is approximately 60% [1]. Recently, advanced clinical diagnostic technologies, including expanded quantitative urine cultures (EQUC), next generation sequencing (NGS) and nucleic acid amplification tests (NAAT) such as quantitative polymerase chain reaction (qPCR) and real time qPCR, have been developed to address the low sensitivity and prolonged turnaround time of results associated with SUCs [2].
While each of these advanced technologies help address specific challenges of SUC, each also has unique impediments to routine clinical use. For example, EQUC methods enable detection of a broader range of viable bacteria and fungi, but do not improve testing turnaround time [3,4,5]. NGS-based testing provides greater sensitivity and accuracy; however, it is not widely used in routine UTI diagnosis [5], as the requirement of complex data analysis and specialized expertise for testing renders NGS-based UTI testing both time and cost-intensive [6]. qPCR-based molecular testing can overcome several of the challenges that SUC presents, including the ability to detect anaerobic, intracellular, and non-bacterial infections, accurate identification of all causative organisms (even those in low concentrations) in a clinical specimen, and with significantly faster turn-around time (often same-day results). Furthermore, a recent meta-analysis reported that qPCR had higher specificity and sensitivity for UTI pathogen detection compared with NGS [7]. However, despite the potential advantages of qPCR, one of the biggest challenges facing adoption of qPCR testing for UTI diagnosis in the clinical setting is its enhanced sensitivity, particularly when applied to a clinical specimen for which contamination with potential uropathogens is not uncommon. In this setting, quantifying the amount of organism recovered in an SUC has historically helped guide interpretation and treatment decisions.
With SUC, a quantitative threshold of ≥105 CFU/mL of bacteria is regarded as clinically significant in freshly voided urine. Bacterial counts between 104 and 105 CFU/mL are generally interpreted according to clinical status. Bacterial counts < 104 CFU/mL are considered to have lower probability of UTI [8]. Recent studies indicate that bacterial counts as low as 102 CFU/mL can cause UTI in symptomatic patients [9,10,11]. Thus, understanding how qPCR results correlate with that of traditional quantitative SUC results would be beneficial in helping to interpret the qPCR result in the context of the patient’s clinical presentations and symptoms, and ultimately, in understanding which pathogens may or may not require treatment.
To address this challenge, we performed real-time qPCR testing on previously cultured urine samples and compared (colony forming unit per milliliters [CFU/mL] versus quantification cycle [Cq]) for each sample to aid in PCR result interpretation and to enhance clinical applicability.
2. Materials and Methods
2.1. Bacterial Strains
Bacterial strains (Table 1) were obtained from ThermoFisher (Lenexa, KS, USA) and were cultured on Tryptic Soy Agar (TSA) (Cat# R01624) or Mueller-Hinton Agar (MHA) (Cat# R111816) purchased from Remel (Lenexa, KS, USA).

2.2. Quantification of Bacterial Load in Urine Samples Using Culture-Based Methods
Contrived urine samples with various bacterial concentrations were prepared as follows. Using bacterial colonies grown overnight on TSA plates, an initial cell suspension equivalent to 0.5 McFarland (1.5 × 108 CFU/mL) was prepared in sterile urine by adjusting the cell density using a densitometer. A series of seven tenfold dilutions (1.5 × 107 to 1.5 × 100) were prepared in sterile urine. For each dilution, 100 µL was inoculated on either TSA or MHA in triplicate and incubated overnight at 37 °C. Colony counts were performed when discernible colonies were present; confluent growth was recorded as “too numerous to count” (TNTC). The experiment was repeated three times to ensure reproducibility.
2.3. qPCR to Determine the Cq Value for Known Bacterial Population Estimate
For each serial dilution, 1.0 mL was aliquoted in a 1.5 mL micro-centrifuge tube and centrifuged at 10,000× g for 10 min. The supernatant was decanted, and the cell pellet resuspended in 200 µL of PrimeStoreTM molecular transport medium (Longhorn Diagnostics, MD, USA). Total nucleic acid was extracted into 50 µL elution buffer using ThermoFisher KingFisher™ Flex (ThermoFisher Scientific, San Jose, CA, USA) system following the manufacturer’s protocol without any modification. Using 8 µL of extracted nucleic acid, qPCR was carried out using the HealthTrackRx OpenArray™ UTI syndromic panel (Denton, TX, USA) (Table 2) in triplicate for each dilution using the protocol described previously [12]. The OpenArray™ cards were run on ThermoFisher QuantStudio™ 12K Flex platform (ThermoFisher Scientific, CA, USA) with the following PCR cycling conditions: initial Enzyme activation at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 15 s followed by annealing/extension at 60 °C. The OpenArray™ technology is a high-throughput nanofluidic platform that uses custom arrays pre-plated with TaqManTM assays for the detection of target pathogens via PCR. The performance of PCR assays with respect to limit of detection, sensitivity and specificity were performed according to well-established Clinical and Laboratory Standards Institute (CLSI) guidelines for multiplex PCR assays.


2.4. Establishing the Cq to CFU/mL Correlation Algorithm
Using results from the contrived urine specimens, and considering culture quantification as the standard, an algorithm was developed to correlate CFU/mL with Cq values as follows: the serially diluted bacteria were plated in triplicate at each dilution. Bacterial colonies were counted on each culture plate and counts were recorded. The average across three plates for each dilution was recorded and multiplied by a factor of ten to give CFU/mL. qPCR was performed in triplicate for each dilution to generate Cq values. The average of the three Cq values for each dilution was calculated. Standard curves for each pathogen target were established by plotting the Cq values against the CFU/mL data for each dilution. A correlational algorithm was then established to define a clinically relevant threshold of 105 CFU/mL based on Cq values obtained through qPCR according to the linear equation obtained through the standard curves.
2.5. Correlation of Cq and CFU in Clinical Specimens
Residual and de-identified clinical urine samples were obtained from ARUP Labs, Utah, USA. Samples were collected over a period of two months in a blind fashion to avoid any bias. No patient information was collected and only the concordance between the microbial culture and PCR results was calculated based on the detection of the pathogens. A study comparing the qualitative results of urine culture and qPCR for the specimen cohort was previously published [13]. In the current study, we retrospectively analyzed the concordance between Cq and CFU/mL in the 168 specimens for which both traditional urine culture data and Cq values using UTI syndromic qPCR were available. Correlation was performed for all bacterial isolates based on their Gram staining properties using the developed algorithm.
2.6. Statistical Analysis
Categorical variables are presented as numerical values (n, %). Error bars wherever presented depict the standard deviation. Statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Analysis, Vienna, Austria).
3. Results
3.1. Estimation of Colony-Forming Units from Serially Diluted Bacterial Cell Suspensions
To quantify bacterial load across serial dilutions, CFU/mL values were log-transformed (Log10) and plotted against the expected cell density based on turbidity measurements (Figure 1a). The dilution series began with a 0.5 McFarland standard, corresponding to 1.5 × 108 CFU/mL.

Figure 1. (a) Log10 CFU/mL for serially diluted bacterial cell suspension. The serially diluted bacteria were plated in triplicate at each dilution. All counts were recorded. The Average across three plates was recorded and multiplied by factor of ten to give CFU/mL. The value CFU/mL was transformed into Log10 value and plotted. The error bars indicate the standard deviation across three experiments of Log10 value of CFU/mL. Error bars represent standard deviation. (b) Open ArrayTM quantification cycle (Cq) values obtained at dilutions 103 to 108 CFU/mL for five bacteria spiked in urine. Sterile urine spiked with bacterial cells with concentrations ranging from 108 CFU/mL to 100 CFU/mL assayed for Cq values using qPCR. Each experiment was performed in triplicate. The data shown here is an average of three independent experiments. Error bars indicate standard deviation across three independent experiments. Error bars represent standard deviation.
Optical density measurements provided additional insight into bacterial concentration. When the expected Log10 (CFU/mL) value was 3.17, the average optical density for Gram-negative bacteria was 2.87 (±0.05), while for Gram-positive bacteria it was 3.02 (±0.03). At culture densities exceeding 105 CFU/mL, colony growth became too confluent for accurate enumeration, and these values were recorded as TNTC and excluded from further analysis.
3.2. Determination Cq Values by Open Array™ Multiplex qPCR
At bacterial cell densities of 104 and 105 CFU/mL, the following Cq values were observed: E. faecalis 27.5 (±1.9) and 26.2 (±2.4), S. aureus 28.1 (±1.3) and 25.7 (±2.4), E. coli 25.4 (±2.7) and 22.2 (±2.7), K. pneumoniae 25.7 (±1.4) and 21.7 (±1.4), P. aeruginosa 25.7 (±1.0) and 22.2 (±0.9), respectively. On average, Gram-positive bacteria showed distinctively different Cq values to that of the Gram-negative bacteria tested. In the case of Gram-negative bacteria at 105 CFU/mL, the average Cq value was 21.8 (±1.7), whereas for Gram-positive bacteria it was 26.5 (±2.4). Similarly, at 104 CFU/mL for Gram-negative bacteria, the average Cq was 25.6 (±1.6), whereas for Gram-positive bacteria it was 27.9 (±1.5) (Figure 1b).
3.3. Cq to CFU/mL Correlation Algorithm
Based on the data from Cq to CFU/mL experiments (Figure 1a,b), a clinically relevant cutoff of 105 CFU/mL was determined on the basis of Cq values generated via qPCR. Per Table 3, for Gram-negative bacteria, Cq values of <23 corresponded with ≥105 CFU/mL and Cq values between 23 and 28 corresponded with <105 CFU/mL. For Gram-positive bacteria, Cq values of <26 corresponded to ≥105 CFU/mL and Cq values between 26 and 30, corresponded with <105 CFU/mL. With respect to the urine culture standards, Cq > 28 for Gram-negative bacteria and Cq > 30 for Gram-positive bacteria corresponded to negative.

We then retrospectively applied the Cq to CFU/mL criteria established using the three Gram-negative (E. coli, K. pneumoniae and P. aeruginosa) and two Gram-positive bacteria (S. aureus and E. faecalis) (Table 3) to the 168 residual clinical samples. Among 138 urine samples that grew Gram-negative pathogens, 116 resulted as ≥105 CFU/mL and among these, 89.6% (n = 104) displayed a Cq value of <23, which directly correlated to 105 CFU/mL range. In addition, of the remaining 12 Gram-negative bacteria that resulted as 105 CFU/mL, six had Cq values greater than 23 were all within the ±2 standard deviation (SD) observed in our study. Of 30 urine specimens that grew Gram-positive bacteria, 15 were quantified as greater than 105 CFU/mL and among those, 11 were below the PCR Cq value of 26. Overall, there was 83.3% agreement (n = 140/168) and 16.6% non-agreement (n = 28/168) between culture CFU/mL and Cq to CFU/mL. For Gram-negative isolates (n = 138), there was 87.6% (121/138) agreement and 12.3% (17/138) disagreement. Whereas, for Gram-positive bacteria (n = 30), the agreement was 63.3% (n = 19/30) and disagreement was 36.6% (n = 11/30) (Table 4).

The average Cq value detected for the five bacteria species used to develop the correlation algorithm all agreed with the proposed threshold values (Table 3). For E. coli the average Cq value detected in cultures reported as >105 CFU/mL was 17.99 (range 12.97 to 27.66). For Klebsiella the average value was 20.82 (range 17.11 to 23.89). The three Pseudomonas isolates gave an average Cq value of 22.61 (range 21.64 to 26.469). Two Enterococcus species detected had an average Cq value of 22.47 (19.94 and 25) and S. aureus Cq values averaged 21.26 (range 14.73 to 28.19). In cultures reported as <105 CFU/mL, thirteen E. coli were detected with an average Cq of 25.26 (range 18.69 to 28.46). The average for Klebsiella Cq values was 27.4 (range 23.51 to 19.25), and Enterococcus, 27 (26 to 29.7). The Cq-CFU/mL threshold algorithm developed as part of this study was also applied on Gram-negative and Gram-positive bacterial pathogens which were not part of the Cq-CFU/mL correlation, but that were detected by the multiplex PCR panel. A total of twenty-one isolates, sixteen Gram-negative and five Gram-positive bacteria were analyzed. Thirteen isolates showed positive agreement between reported CFU/mL values and CFU/mL values derived using the threshold values. All but one Proteus isolate (1/13) showed positive agreement for specimens reported as greater than 105 CFU/mL. Four S. agalactiae (GBS), one Enterobacter and two Citrobacter isolates were reported with CFU/mL values less than 105 CFU/mL which, following application of our proposed threshold values, the Cq values detected would have correlated to a CFU/mL value greater than 105 CFU/mL (Table 4, Figure 2).

Figure 2. Cq-CFU/mL clinical validation. Cq values obtained from qPCR tests were compared to the previously tested urine samples via microbial culture. A Cq cutoff of ≤23 (for Gram-negative) and ≤26 (for Gram-positive) was used to establish correlation with culture results above or below 105 CFU/mL. The figure summarizes agreement/non-agreement between qPCR and microbial culture techniques reported in Table 4.
4. Discussion
To improve the clinical efficacy of multiplex qPCR-based UTI detection and to provide a tool for clinicians to reliably interpret UTI PCR results in relation to traditional culture results, this study aimed to correlate the multiplex UTI PCR values (Cq) with colony counts (CFU/mL) of urine cultures. Unlike molecular panels for other infectious syndromes, fewer studies have compared multiplex PCR to traditional urine culture for diagnosing UTIs [14,15,16]. For comparison, 100,000 CFU/mL (105) was set as the target comparator as this value is widely considered a clinical diagnostic cutoff for diagnosing UTIs in symptomatic patients [5].
The results from our study showed a CFU/mL value of ≥105 correlated with average Cq values below 22.5 for all Gram-negative bacteria assessed, whereas for Gram-positive bacteria the Cq value that best correlated to ≥105 CFU/mL was 26.5 (Table 3). Our results are in agreement with previously published studies wherein multiplex PCR-based detection of UTI-causing pathogens was directly compared to those detected via culture in a clinical setting [17]. In a similar comparative analysis of Cq and CFU/mL values for bacterial pathogens in bronchial samples, Burillo and co-workers reported 24.7 as the Cq value that best corresponded to 105 CFU/mL. The authors also described 104 CFU/mL as corresponding to an average Cq value of 26.9 for Gram-negative bacteria [18].
The derived semi-quantitative thresholds correlating Cq values to urine culture CFU/mL values were validated using a collection of clinical specimens previously submitted for urine culture and reported [13]. Based on the results of our Cq-CFU/mL study (Table 3), we used a Cq value of 23 for Gram-negative bacteria and Cq of 26 for Gram-positive bacteria as the semi-quantitative criteria to approximate a CFU/mL of ≥105. After converting Cq values to CFU/mL for previously tested urine isolates, we looked at the agreement between the two sets of CFU/mL values. The percent agreement between the results obtained after applying the threshold values was 83.3% (140/168) for the five bacterial strains used in this study (Table 4). Despite the overall high agreement, we observed 36.6% disagreement for Gram-positive bacteria. This can be attributed to differences in overall PCR efficiency, assay design and the small number of Gram-positive strains utilized to derive the Cq value scale which underscores the need for further studies with additional bacterial strains and qPCR assays to establish a more robust interpretation scale.
A major concern with PCR-based testing is the detection of pathogens in samples that are deemed culture negative [14,15,16], suggesting that PCR may be too sensitive a technique for reliably reporting clinically relevant levels of uropathogens. In general, the decision-making process of appropriate diagnostic tests for UTIs (based on clinical symptoms) as well as result interpretation for timely treatment remains a challenge [19]. Furthermore, the high sensitivity of molecular tests in comparison to traditional culture techniques complicates clinical interpretation of the results and remains a significant barrier in the widespread adoption of PCR for the detection of UTIs. Since PCR can detect nucleic acid material from non-viable and degraded pathogens, a positive PCR result may not reflect a true UTI even in symptomatic patients [20]. Similarly, in cases of a culture-negative but PCR-positive diagnosis, a clear distinction between infection and colonization cannot be made without further research into the clinical validity of the quantitative PCR test [17]. A study comprising 220 women with UTI symptoms demonstrated that 95.9% samples were positive for E. coli via PCR versus 80.9% cultures positive for any UTI-causing pathogen [17]. In our study, among the previously tested urine culture specimens, those reported as negative by culture were also analyzed. The multiplex UTI PCR panel included pathogens not readily detected in traditional culture, and several were observed in the study. Notably, there were 59 culture-negative specimens where E. coli was detected via PCR [13]. Upon applying our proposed threshold values for Cq-CFU/mL, 93.2% (n = 55/59) of these PCR-positive E. coli samples were interpreted to be less than 105 CFU/mL and only four had Cq values that would have been reported (≥105 CFU/mL). These findings suggest that our proposed semi-quantitative criteria, when applied to Cq values from patient specimens, reliably correlates to CFU/mL cutoff values used in the clinical diagnosis of UTIs and may help delineate pathogens from non-pathogenic and/or non-viable colonizers.
Previous studies have argued that PCR is a reliable and beneficial replacement for traditional urine culture [17,18,21]. Our study shows that a robust correlation between Cq and CFU/mL results exists, and the correlation can be effectively incorporated into result reports to assist in patient diagnosis and to enable more targeted approaches to antimicrobial therapy.
The implementation of syndromic PCR panels has had a significant impact on rapid disease diagnosis [22]. In support of NAATs, it has been opined that large multiplex PCR panels should be the first-line tests for detection of respiratory and intestinal pathogens [23]. A single-center, 150-patient study showed the standard urine culture (at 105 CFU/mL) displayed poor sensitivity and missed 67% of all detected UTI-causing pathogens, indicating a need for more sensitive methods for pathogen detection in urine specimens [3]. The increasing demand for high-throughput tests that can detect a range of diverse pathogens has further placed multiplex testing at the forefront of options sought by the modern clinical laboratory [2,24]. This testifies to the growing importance of multiplex PCR tests as an effective tool for uropathogen detection in clinical samples. Despite the increased sensitivity and specificity, a major disadvantage of PCR is its limitation of detecting only known resistance genes and the technology in its current format cannot be used for assessing true antibiotic sensitivity of a detected pathogen as opposed to established phenotypic susceptibility testing methods. However, when interpreted in conjunction with the clinical presentation and examination, qPCR tests may streamline testing, optimize antibiotic use and potentially improve patient outcomes [25,26].
We recognize that the current study has limitations. Firstly, the number of pathogens used to validate our Cq-CFU/mL threshold algorithm is limited. Further studies using more bacterial strains, particularly Gram-positive species, are currently being performed to support the observations made in this study. Secondly, the clinical validation on clinical samples was performed on residual samples. Another limitation of our study is the non-standardized nature of the PCR assay used, and the results cannot be universally utilized without incorporating multi-site testing. To further demonstrate the true clinical effectiveness of our correlation algorithm, prospective studies with defined patient populations are needed wherein urine culture is compared to the Open ArrayTM multiplex PCR panel simultaneously which would enhance the observations made in this study.
5. Conclusions
Molecular testing for UTIs, when compared to traditional culture techniques, presents advantages with respect to increased sensitivity and rapid turnaround time for results. However, widespread acceptance of this methodology is hampered by limitations of PCR-based results for establishing clinically relevant cutoffs of the detected uropathogens. Our results establish a clear agreement between the PCR-generated Cq value and the traditional CFU/mL value generated via microbial culture. Validation of our semi-quantitative interpretive scale using clinical samples demonstrates that PCR Cq values correspond with established standard of care quantitative culture thresholds, providing physicians with valuable additional information to aid in interpreting qPCR results for the diagnosis of UTIs.
References
- Yoo, J.-J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; Kim, W.B.; et al. Urinary Microbiome Characteristics in Female Patients with Acute Uncomplicated Cystitis and Recurrent Cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef]
- Szlachta-McGinn, A.; Douglass, K.M.; Chung, U.Y.R.; Jackson, N.J.; Nickel, J.C.; Ackerman, A.L. Molecular Diagnostic Methods Versus Conventional Urine Culture for Diagnosis and Treatment of Urinary Tract Infection: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2022, 44, 113–124. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Wolfe, A.J.; Brubaker, L. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef]
- Deen, N.S.; Ahmed, A.; Tasnim, N.T.; Khan, N. Clinical Relevance of Expanded Quantitative Urine Culture in Health and Disease. Front. Cell. Infect. Microbiol. 2023, 13, 1210161. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Deebel, N.; Casals, R.; Dutta, R.; Mirzazadeh, M. A New Gold Rush: A Review of Current and Developing Diagnostic Tools for Urinary Tract Infections. Diagnostics 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, I.; Aschbacher, R.; Pagani, E. Current Uses and Future Perspectives of Genomic Technologies in Clinical Microbiology. Antibiotics 2023, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qi, S.; Sun, Y.; Zheng, X. Comparison of Polymerase Chain Reaction and Next-Generation Sequencing with Conventional Urine Culture for the Diagnosis of Urinary Tract Infections: A Meta-analysis. Open Med. 2024, 19, 20240921. [Google Scholar] [CrossRef]
- Karah, N.; Rafei, R.; Elamin, W.; Ghazy, A.; Abbara, A.; Hamze, M.; Uhlin, B.E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings. Diagnostics 2020, 10, 832. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norby, S.R. Urinary Tract Infections: Disease Panorama and Challenges. J. Infect. Dis. 2001, 183, S1–S4. [Google Scholar] [CrossRef]
- Hooton, T.M.; Roberts, P.L.; Cox, M.E.; Stapleton, A.E. Voided Midstream Urine Culture and Acute Cystitis in Premenopausal Women. N. Engl. J. Med. 2013, 369, 1883–1891. [Google Scholar] [CrossRef]
- Kunin, C.M.; White, L.V.; Hua, T.H. A Reassessment of the Importance of “Low-Count” Bacteriuria in Young Women with Acute Urinary Symptoms. Ann. Intern. Med. 1993, 119, 454–460. [Google Scholar] [CrossRef]
- Singh, V.; Reddy, J.; Granger, J. A Survey of Viral-Bacterial Co-infection in Respiratory Samples Using Multiplex Real-Time PCR. J. Infect. Dis. Ther. 2019, 7, 400. [Google Scholar]
- Upadhyay, P.; Surar, F.; Kim, G.; Reddy, J.; Shakir, S.; Alexander, B.D.; Hanson, K.; Singh, V. Comparative Analysis of the Detection of UTI Pathogens via Culture Method and the Open Array-Nanofluidic Real Time PCR Method. Open Forum Infect. Dis. 2022, 9, ofac492. [Google Scholar] [CrossRef]
- Cybulski, Z.; Schmidt, K.; Grabiec, A.; Talaga, Z.; Bociąg, P.; Wojciechowicz, J.; Roszak, A.; Kycler, W. Usability Application of Multiplex Polymerase Chain Reaction in the Diagnosis of Microorganisms Isolated from Urine of Patients Treated in Cancer Hospital. Radiol. Oncol. 2013, 47, 296–303. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, A.; Roorda, L.; Bosman, G.; Ossewaarde, J.M. Molecular Diagnosis of Urinary Tract Infections by Semi-Quantitative Detection of Uropathogens in a Routine Clinical Hospital Setting. PLoS ONE 2016, 11, e0150755. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2020, 136, 119–126. [Google Scholar] [CrossRef]
- Heytens, S.; De Sutter, A.; Coorevits, L.; Cools, P.; Boelens, J.; Van Simaey, L.; Christiaens, T.; Vaneechoutte, M.; Claeys, G. Women with Symptoms of a Urinary Tract Infection but a Negative Urine Culture: PCR-Based Quantification of Escherichia coli Suggests Infection in Most Cases. Clin. Microbiol. Infect. 2017, 23, 647–652. [Google Scholar] [CrossRef]
- Burillo, A.; Marín, M.; Cercenado, E.; Ruiz-Carrascoso, G.; Pérez-Granda, M.J.; Oteo, J.; Bouza, E.; Floto, A.R. Evaluation of the Xpert Carba-R (Cepheid) Assay Using Contrived Bronchial Specimens from Patients with Suspicion of Ventilator-Associated Pneumonia for the Detection of Prevalent Carbapenemases. PLoS ONE 2016, 11, e016847. [Google Scholar] [CrossRef]
- Medina-Bombardó, D.; Jover-Palmer, A. Does Clinical Examination Aid in the Diagnosis of Urinary Tract Infections in Women? A Systematic Review and Meta-Analysis. BMC Fam. Pract. 2011, 12, 111. [Google Scholar] [CrossRef]
- Lehmann, L.E.; Hauser, S.; Malinka, T.; Klaschik, S.; Weber, S.U.; Schewe, J.C.; Stüber, F.; Book, M. Rapid Qualitative Urinary Tract Infection Pathogen Identification by SeptiFast Real-Time PCR. PLoS ONE 2011, 6, e17146. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Dumkow, L.E.; Worden, L.J.; Rao, S.N. Syndromic Diagnostic Testing: A New Way to Approach Patient Care in the Treatment of Infectious Diseases. J. Antimicrob. Chemother. 2021, 76, iii4–iii11. [Google Scholar] [CrossRef]
- Schreckenberger, P.C.; McAdam, A.J. Point-Counterpoint: Large Multiplex PCR Panels Should Be First-Line Tests for Detection of Respiratory and Intestinal Pathogens. J. Clin. Microbiol. 2015, 53, 3110–3115. [Google Scholar] [CrossRef]
- Huang, H.S.; Tsai, C.L.; Chang, J.; Hsu, T.C.; Lin, S.; Lee, C.C. Multiplex PCR System for the Rapid Diagnosis of Respiratory Virus Infection: Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2018, 24, 1055–1063. [Google Scholar] [CrossRef]
- Kelly, B.N. UTI Detection by PCR: Improving Patient Outcomes. J. Mass Spectrom. Adv. Clin. Lab. 2023, 28, 60–62. [Google Scholar] [CrossRef]
- Elia, J.; Hafron, J.; Holton, M.; Ervin, C.; Hollander, M.B.; Kapoor, D.A. The Impact of Polymerase Chain Reaction Urine Testing on Clinical Decision-Making in the Management of Complex Urinary Tract Infections. Int. J. Mol. Sci. 2024, 25, 6616. [Google Scholar] [CrossRef]
References
- Yoo, J.-J.; Shin, H.B.; Song, J.S.; Kim, M.; Yun, J.; Kim, Z.; Lee, Y.M.; Lee, S.W.; Lee, K.W.; Kim, W.B.; et al. Urinary Microbiome Characteristics in Female Patients with Acute Uncomplicated Cystitis and Recurrent Cystitis. J. Clin. Med. 2021, 10, 1097. [Google Scholar] [CrossRef]
- Szlachta-McGinn, A.; Douglass, K.M.; Chung, U.Y.R.; Jackson, N.J.; Nickel, J.C.; Ackerman, A.L. Molecular Diagnostic Methods Versus Conventional Urine Culture for Diagnosis and Treatment of Urinary Tract Infection: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2022, 44, 113–124. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Wolfe, A.J.; Brubaker, L. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef]
- Deen, N.S.; Ahmed, A.; Tasnim, N.T.; Khan, N. Clinical Relevance of Expanded Quantitative Urine Culture in Health and Disease. Front. Cell. Infect. Microbiol. 2023, 13, 1210161. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Deebel, N.; Casals, R.; Dutta, R.; Mirzazadeh, M. A New Gold Rush: A Review of Current and Developing Diagnostic Tools for Urinary Tract Infections. Diagnostics 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, I.; Aschbacher, R.; Pagani, E. Current Uses and Future Perspectives of Genomic Technologies in Clinical Microbiology. Antibiotics 2023, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qi, S.; Sun, Y.; Zheng, X. Comparison of Polymerase Chain Reaction and Next-Generation Sequencing with Conventional Urine Culture for the Diagnosis of Urinary Tract Infections: A Meta-analysis. Open Med. 2024, 19, 20240921. [Google Scholar] [CrossRef]
- Karah, N.; Rafei, R.; Elamin, W.; Ghazy, A.; Abbara, A.; Hamze, M.; Uhlin, B.E. Guideline for Urine Culture and Biochemical Identification of Bacterial Urinary Pathogens in Low-Resource Settings. Diagnostics 2020, 10, 832. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norby, S.R. Urinary Tract Infections: Disease Panorama and Challenges. J. Infect. Dis. 2001, 183, S1–S4. [Google Scholar] [CrossRef]
- Hooton, T.M.; Roberts, P.L.; Cox, M.E.; Stapleton, A.E. Voided Midstream Urine Culture and Acute Cystitis in Premenopausal Women. N. Engl. J. Med. 2013, 369, 1883–1891. [Google Scholar] [CrossRef]
- Kunin, C.M.; White, L.V.; Hua, T.H. A Reassessment of the Importance of “Low-Count” Bacteriuria in Young Women with Acute Urinary Symptoms. Ann. Intern. Med. 1993, 119, 454–460. [Google Scholar] [CrossRef]
- Singh, V.; Reddy, J.; Granger, J. A Survey of Viral-Bacterial Co-infection in Respiratory Samples Using Multiplex Real-Time PCR. J. Infect. Dis. Ther. 2019, 7, 400. [Google Scholar]
- Upadhyay, P.; Surar, F.; Kim, G.; Reddy, J.; Shakir, S.; Alexander, B.D.; Hanson, K.; Singh, V. Comparative Analysis of the Detection of UTI Pathogens via Culture Method and the Open Array-Nanofluidic Real Time PCR Method. Open Forum Infect. Dis. 2022, 9, ofac492. [Google Scholar] [CrossRef]
- Cybulski, Z.; Schmidt, K.; Grabiec, A.; Talaga, Z.; Bociąg, P.; Wojciechowicz, J.; Roszak, A.; Kycler, W. Usability Application of Multiplex Polymerase Chain Reaction in the Diagnosis of Microorganisms Isolated from Urine of Patients Treated in Cancer Hospital. Radiol. Oncol. 2013, 47, 296–303. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, A.; Roorda, L.; Bosman, G.; Ossewaarde, J.M. Molecular Diagnosis of Urinary Tract Infections by Semi-Quantitative Detection of Uropathogens in a Routine Clinical Hospital Setting. PLoS ONE 2016, 11, e0150755. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2020, 136, 119–126. [Google Scholar] [CrossRef]
- Heytens, S.; De Sutter, A.; Coorevits, L.; Cools, P.; Boelens, J.; Van Simaey, L.; Christiaens, T.; Vaneechoutte, M.; Claeys, G. Women with Symptoms of a Urinary Tract Infection but a Negative Urine Culture: PCR-Based Quantification of Escherichia coli Suggests Infection in Most Cases. Clin. Microbiol. Infect. 2017, 23, 647–652. [Google Scholar] [CrossRef]
- Burillo, A.; Marín, M.; Cercenado, E.; Ruiz-Carrascoso, G.; Pérez-Granda, M.J.; Oteo, J.; Bouza, E.; Floto, A.R. Evaluation of the Xpert Carba-R (Cepheid) Assay Using Contrived Bronchial Specimens from Patients with Suspicion of Ventilator-Associated Pneumonia for the Detection of Prevalent Carbapenemases. PLoS ONE 2016, 11, e016847. [Google Scholar] [CrossRef]
- Medina-Bombardó, D.; Jover-Palmer, A. Does Clinical Examination Aid in the Diagnosis of Urinary Tract Infections in Women? A Systematic Review and Meta-Analysis. BMC Fam. Pract. 2011, 12, 111. [Google Scholar] [CrossRef]
- Lehmann, L.E.; Hauser, S.; Malinka, T.; Klaschik, S.; Weber, S.U.; Schewe, J.C.; Stüber, F.; Book, M. Rapid Qualitative Urinary Tract Infection Pathogen Identification by SeptiFast Real-Time PCR. PLoS ONE 2011, 6, e17146. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An Introduction to the Epidemiology and Burden of Urinary Tract Infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Dumkow, L.E.; Worden, L.J.; Rao, S.N. Syndromic Diagnostic Testing: A New Way to Approach Patient Care in the Treatment of Infectious Diseases. J. Antimicrob. Chemother. 2021, 76, iii4–iii11. [Google Scholar] [CrossRef]
- Schreckenberger, P.C.; McAdam, A.J. Point-Counterpoint: Large Multiplex PCR Panels Should Be First-Line Tests for Detection of Respiratory and Intestinal Pathogens. J. Clin. Microbiol. 2015, 53, 3110–3115. [Google Scholar] [CrossRef]
- Huang, H.S.; Tsai, C.L.; Chang, J.; Hsu, T.C.; Lin, S.; Lee, C.C. Multiplex PCR System for the Rapid Diagnosis of Respiratory Virus Infection: Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2018, 24, 1055–1063. [Google Scholar] [CrossRef]
- Kelly, B.N. UTI Detection by PCR: Improving Patient Outcomes. J. Mass Spectrom. Adv. Clin. Lab. 2023, 28, 60–62. [Google Scholar] [CrossRef]
- Elia, J.; Hafron, J.; Holton, M.; Ervin, C.; Hollander, M.B.; Kapoor, D.A. The Impact of Polymerase Chain Reaction Urine Testing on Clinical Decision-Making in the Management of Complex Urinary Tract Infections. Int. J. Mol. Sci. 2024, 25, 6616. [Google Scholar] [CrossRef]